Cipher Pharmaceuticals - Piclidenoson
An overview of piclidenoson's competitive positioning within the plaque psoriasis landscape
If you’ve been following the blog, you’ve seen I’ve been putting out a fair bit of content related to Cipher Pharmaceuticals. In the interests of full disclosure, Cipher Pharmaceuticals has become a significant part of my personal wealth as it now comprises over half of my net worth at this point in time.
My first publication was on June 26th, 2023, when the stock was trading at $3.52/CAD per share.
Since then, a number of important events have taken place which I’ve detailed in subsequent posts. Today the shares are trading at $6.24. While this is a fairly robust performance, it’s my personal view this is not adequately reflective of the potential value of Cipher’s two primary candidate assets (MOB-015 & CF101) which have been licensed from Moberg Pharma & Can-Fite Biopharma.
The focus of this post is to frame the opportunity set of piclidenoson for the treatment of moderate to severe plaque psoriasis. Paired with my writeup on Moberg, the two form a 1000-yard view of the positioning of the two assets for Cipher Pharmaceuticals.
While Cipher claims to have another pipeline asset, it remains in preclinical stages to this day. It’s a ‘tattoo removal cream’, which on its surface sounds like a fantastic product as laser tattoo removal requires labor and is moderately painful. But the product also seems a bit like hogwash.
The rights to the product were licensed from a grad student several years ago for a couple thousand dollars under different management. The only writing I can find on the product outside of disclosures made by Cipher Pharmaceuticals themselves is from a school newspaper from 2015, which briefly describes how the product works and that its been tested on tattooed pig ears.
Bringing this back to the topic of the post; this has been by far the most difficult collection of research I’ve put together for the company. In no small part due to an extensive number of existing therapeutic options and modalities for the treatment of plaque psoriasis. But also because I didn’t simply want to info dump a bunch of needless minutiae into the post which is quite easy to do when you’re new to a complicated subject.
Those looking for a comprehensive overview of the plaque psoriasis medical landscape should read the Joint American Academy of Dermatology National Psoriasis Foundation guidelines of care for the management of psoriasis. The documentation is free and open source. Further, UpToDate is a primary resource pertaining to treatment guidelines, though you will need someone who has access to view the documents.
My goal in creating this was to make for an abbreviated guide that’s understandable to the motivated layman (as that’s what I am) in an effort to understand the landscape so that we may think clearly about one of cipher pharmaceuticals primary growth catalysts: piclidenoson.
So, if Cipher Pharmaceuticals is currently in your portfolio, consider subscribing. These clinical stage assets are very much worth understanding as they quite literally set the stage for future outcomes.
For those of you who are already subscribed, I thank you for your support and look forward to bringing you additional research in the future.
“There is a high market need for a safe and efficacious drug for the treatment of adolescents who suffer from psoriasis. There are a couple of small molecule or biological drugs on the market in use for adolescents with psoriasis, all have safety issues and are not satisfactory regarding the efficacy.”
- Can-Fite
“Preparatory Work for Pivotal Phase III Psoriasis Study; Can Fite Received Green Light from FDA and EMA— Following positive responses from the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for its registration plan and pivotal Phase III study protocol for Piclidenoson in the treatment of moderate to severe psoriasis, the Company is preparing for study initiation. The FDA requested two Phase III studies and also encouraged the Company to enroll adolescent patients due to Piclidenoson’s strong safety profile demonstrated over its development history and prior clinical studies. Can-Fite has submitted to the FDA a pediatric plan to allow the registration of Piclidenoson for the treatment of adolescents. Inclusion of adolescents for the psoriasis Indication is expected to broaden the market.”
- Can-Fite
Below is a flow chart of the medications prescribed for the treatment of plaque psoriasis. Our focus is on the lower right half of the chart.
Predominantly, the first-line systemic oral medications treatment options for moderate to severe plaque psoriasis is methotrexate, ciclosporin, acitretin and apremilast. All four have issues I will be discussing shortly. Some of these can also take the form of injections.
There is another oral drug that has recently been approved called deucravacitinib in Canada that I will be touching on later in this post. Events surrounding deucravacitnib involving Canada's drug and health technology agency likely represent the bear argument against piclidenoson pertaining to Cipher Pharma’s future commercialization efforts should the drug be approved.
I will not be discussing topical treatments in this post as they’re seldom adequate to control moderate to severe plaque psoriasis by themselves and often they’re used in tandem with other treatment methods - especially phototherapy.
Narrow band UVB phototherapy is one of the most commonly used treatment options for plaque psoriasis, however its use in a clinical setting has been declining in the United States (and likely throughout the world). This trend is attributed to innovation in pharmaceuticals and administrative/logistical difficulties of utilizing a phototherapy booth.
Pictured: Model inside a NB-UVB phototherapy booth
There have been investigations into whether NB-UVB phototherapy results in an increased prevalence of skin cancer but results thus far are inconclusive of any relation. This is not the same case with PUVA therapy, which has been connected to an increased incidence of squamous cell cancer, although I expect this is largely attributed to the differences in the range of wavelengths a patient is exposed to (320-400nm in PUVA versus 311–312nm in NB-UVB phototherapy)
Biological assets (those highlighted in the flow chart above) take the form of injections or infusions - more on this later.
While esters of fumaric acid have been used to treat moderate to severe plaque psoriasis in children, its use is off label. It’s also unlisted as an available systemic treatment on the Canadian psoriasis network, and no, there’s no ‘atypical systemic drugs’ category.
Guidelines for how medications are prescribed for the treatment of childhood plaque psoriasis are spelled out by S2K guidelines (S2K guidelines are developed by a committee of specialists in the medical field in question. The recommendations made by the guidelines are consensus-based. A description of what these guidelines mean can be found here.): “only if the treatment response to nonbiological systemic therapy is absent, contraindicated, or not tolerated, may approved biological medicine be considered.”
In my view this is important. What this effectively does, should the drug be approved, is that a patient works through a check list of non-biological systemic agents with their physician prior to the utilization of biologics. A flow chart for drug type utilization, if you will.
What this means in practice is that if you simply take the most efficacious drugs in terms of patient response to medications (which are biologics), you will misinterpret the competitive positioning of any new systemic oral therapy in moderate to severe plaque psoriasis in pediatric populations.
Further, methotrexate, ciclosporin, acitretin, & apremilast often cause issues which result in the escalation of a patient’s prescriptions from non-biological systemic treatments to biological therapies. It’s this patient set which piclidenoson has an opportunity to capture in both pediatric and adult populations should they respond to treatment from piclidenoson.
Readers note: While I was unable to find anything as explicit as the above, it’s my impression different criteria are used in the escalation from nonbiological systemic therapies to biologics in adults. If I find explicit criteria outlining this process in the future, I will provide an update.
Patient Survey
From the “Evolution of Patient Perceptions of Psoriatic Disease published in 2021: Results from the Understanding Psoriatic Disease Leveraging Insights for Treatment (UPLIFT) Survey,” under the section titled “Patient and Dermatologist Perceptions of Treatment Satisfaction” in the results section, you will find this screen shot pertaining to data of oral medications.
For the four primary oral medications, I’ve linked to material which fully describes each medication. The table for contraindications for all four medications is found HERE. I highly encourage review of this table - to include contraindications, it lists necessary lifestyle changes and drug interactions for each medication. The list is extensive. I touch more closely on convenience later in this post.
Methotrexate -
Side effects - The incidence of adverse reactions associated with MTX treatment of psoriasis is estimated at approx. 78%.
Cyclosporine -
Side effects - The percentage of patients who discontinued treatment because of side effects rose from a mean +/- SD of 14% +/- 2.4% at 12 months to 41% +/- 6.7% at 48 months.
Acitretin -
Side effects: Common side effects include cheilitis (inflammation of the lips) and alopecia (balding).
Note: Women who take Acitretin must not get pregnant until 3 years after discontinuing its use. The birth defects associated with its use during pregnancy are catastrophic to a fetus. Similarly, breast a woman cannot breast feed an infant while taking Acitretin.
Apremilast -
Side effects: Nausea, upper respiratory infection, headache, and weight loss.
Clinical Data & Safety of Piclidenoson in Humans -
Piclidenoson Clinical Data - see section 3.2 under “Piclidenoson in Psoriasis”
Molecules | Free Full-Text | Drugs Targeting the A3 Adenosine Receptor: Human Clinical Study Data (mdpi.com). Read this.
If your eyes beginning to glaze over, take a break. Because you absolutely need to read the information from the link above.
“Rest Here Weary Traveler, For Great Adventures Lie Ahead”
Phase II Study - link above
Phase II/III Study - link above
“The efficacy for apremilast was found to plateau at 16 weeks with respect to PASI-75 (response rate of approximately 30%), whereas piclidenoson showed no visible plateau at 32 weeks with respect to PASI-75 (response rate of 35.3%). Similarly, piclidenoson was numerically superior to apremilast with respect to PASI-50 and PASI-90 rates at 32 weeks [45].”
Phase III Comfort Study - see link
The study data show that patients treated with oral Piclidenoson 2 mg or 3 mg twice daily, had clinically equivalent efficacy responses. At week 16, patients receiving Piclidenoson 3mg demonstrated statistically significant improvement when compared with placebo, as measured by the Psoriasis Area and Severity Index (PASI) 75 response: Piclidenoson 3mg: 9.7% vs. placebo: 2.6% (P< 0.04). Secondary endpoint parameters at week 32 comparing Piclidenoson to the active control drug, Otezla, revealed inferiority with respect to PASI 75 (17% vs. 26.2%, respectively) and PASI 50 (34.1% vs. 49.5%, respectively), but revealed superiority of Piclidenoson as compared to Otezla in the Psoriasis Disability Index (PDI) (20.5% vs. 10.3%, respectively, P<0.05). A linear increase in the response of patients to Piclidenoson was achieved along the study period, on week 48 reaching PASI 50 in 90% of patients, PASI 90 in 10% of patients and PDI improvement in 60% of patients.
Piclidenoson had an excellent safety profile overlapping that of the placebo treated patients, showing a better safety profile when compared to Otezla (Apremilast).
“Based on Piclidenoson’s safety and efficacy data revealed in this trial, we plan to approach the U.S. FDA and the European EMA with a protocol for a pivotal Phase III study for drug approval and registration,” stated Can-Fite CEO, Dr. Pnina Fishman.
Recall the statement located in Can-Fite’s announcement at the beginning of this article.
“The FDA requested two Phase III studies and also encouraged the Company to enroll adolescent patients due to Piclidenoson’s strong safety profile demonstrated over its development history and prior clinical studies.”
But why is piclidenoson resulting in a lack of side effects relative to other approved plaque psoriasis drugs which can do any number of things, like suppress the immune system, cause rash, fever, yeast infections, dizziness, nausea, fatigue, diarrhea, headache, cirrhosis, loss of appetite, reactivation of herpes simplex virus & herpes zoster, depression, and a number of other side effects.
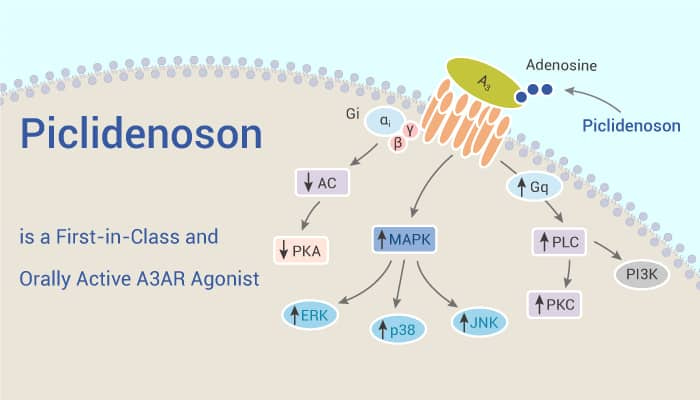
Like all things, it’s because of how it works.
The A3 adenosine receptor (A3AR) is overexpressed in pathological human cells. Piclidenoson and namodenoson are A3AR agonists with high affinity and selectivity to A3AR. Both induce apoptosis of cancer and inflammatory cells via a molecular mechanism entailing deregulation of the Wnt and the NF-κB signaling pathways.
The description in plain English is that for the most part healthy cells, for lack of a better word, are “invisible” to the drug as there is a unique protein inside the plaques which the drug binds to with high affinity and specificity, inhibiting the production of the specific proteins which cause inflammation. This leads into a major component of why piclidenoson has safety benefits over some of the most common/efficacious drugs for the treatment of plaque psoriasis.
Plaque psoriasis is an autoimmune disease.
So, what a bunch of smart scientists did was design a bunch of different drugs which target and inhibit the parts of a patient’s immune system which are causing the inflammation. This is referred to as ‘immune suppression’, and it’s not something physicians take lightly when prescribing a medication. This feature of cyclosporine is one reason why treatment cycles must remain abbreviated.
While the introduction above only details biologics - the same is true of 3 of the 4 oral medications I’ve listed in this piece. The only oral drug which doesn’t act as an immune suppressant in some fashion is Acitretin, which has wonderful side effects like hair loss in 50-75% of people which take the medication.
To this end, it appears there could potentially be a large existing white space within the market for oral drugs in the moderate to severe plaque psoriasis market which can help maintain patient symptoms without conferring as many side effects. The same sentiment is repeated in a presentation from management at Can-Fite in this video.
Moving on to another avenue of research I pursued in writing this…
There is no passion so contagious as that of fear.
— Michel de Montaigne
The fear of needles: A systematic review and meta-analysis
“119 original research articles which are included in this review, of which 35 contained sufficient information for meta-analysis. The majority of children exhibited needle fear, while prevalence estimates for needle fear ranged from 20-50% in adolescents and 20–30% in young adults. In general, needle fear decreased with increasing age. Both needle fear and needle phobia were more prevalent in females than males. Avoidance of influenza vaccination because of needle fear occurred in 16% of adult patients, 27% of hospital employees, 18% of workers at long-term care facilities, and 8% of healthcare workers at hospitals. Needle fear was common when undergoing venipuncture, blood donation, and in those with chronic conditions requiring injection.”
“A large sample of children having immunizations, the self-reported fear of needles was scored as strong in 68% of children aged 6 to 8 years, in 65% of children aged 9 to 12 years, and in 51% of children aged 13 to 17 years (Taddio et al., 2012)”
Survey of the prevalence of immunization non-compliance due to needle fears in children and adults
“Needle fear was the primary reason for immunization non-compliance for 7% and 8% of parents and children, respectively.”
These non-compliance rates with immunization are roughly 2x that of the % of the population which suffer from Blood Injury and Injection Phobia (BII), which has strong genetic heritability and lifetime prevalence (~61% of individuals with BII phobia have one or more family members with the phobia and largely persists throughout a person’s life).
BII is one of the most common heritable psychiatric disorders in the world.
Persistence and Inheritance of Blood Injury and Injection Phobia
Bringing this to Plaque Psoriasis in adolescents.
The numbers used in the plot were calculated via the lower range of the existing incidence of the two conditions amongst the general population (1% of children with PS and 3% with BII) in order to generate a conservative number of patients to which the process of administering biologics proves difficult.
These numbers were arrived at by taking the prevalence of each condition and multiplying them together. I’m not great at statistics, but I think this is roughly how you’re supposed to get to these values.
In addition, moderate to severe plaque psoriasis represent approximately ~30% of cases, making the final number of estimated patients ~529.
While this number may seem quite low at first glance the real numbers are likely higher as:
The incident rate of BII phobia is substantially higher in children than rates found in the general population. Nearly 20% of children at one point or another qualify as having BII, bringing the estimate to ~3,000.
Avoidance rates to immunization and medical treatments attributed to needle fear are higher than the incident rates of BII - remember, BII is a heritable pathological psychiatric disorder characterized by fainting, bradycardia, and intense anxiety when presented with a trigger such as needles, so these numbers disqualify simply avoiding treatment due to needle fear.
With these two considerations in mind, I would intuitively guess the applicable population set is closer to ~5,000 due to needle fear/avoidance in pediatrics & some multiple of that in adult populations (simply due to greater total case numbers of plaque psoriasis in adults).
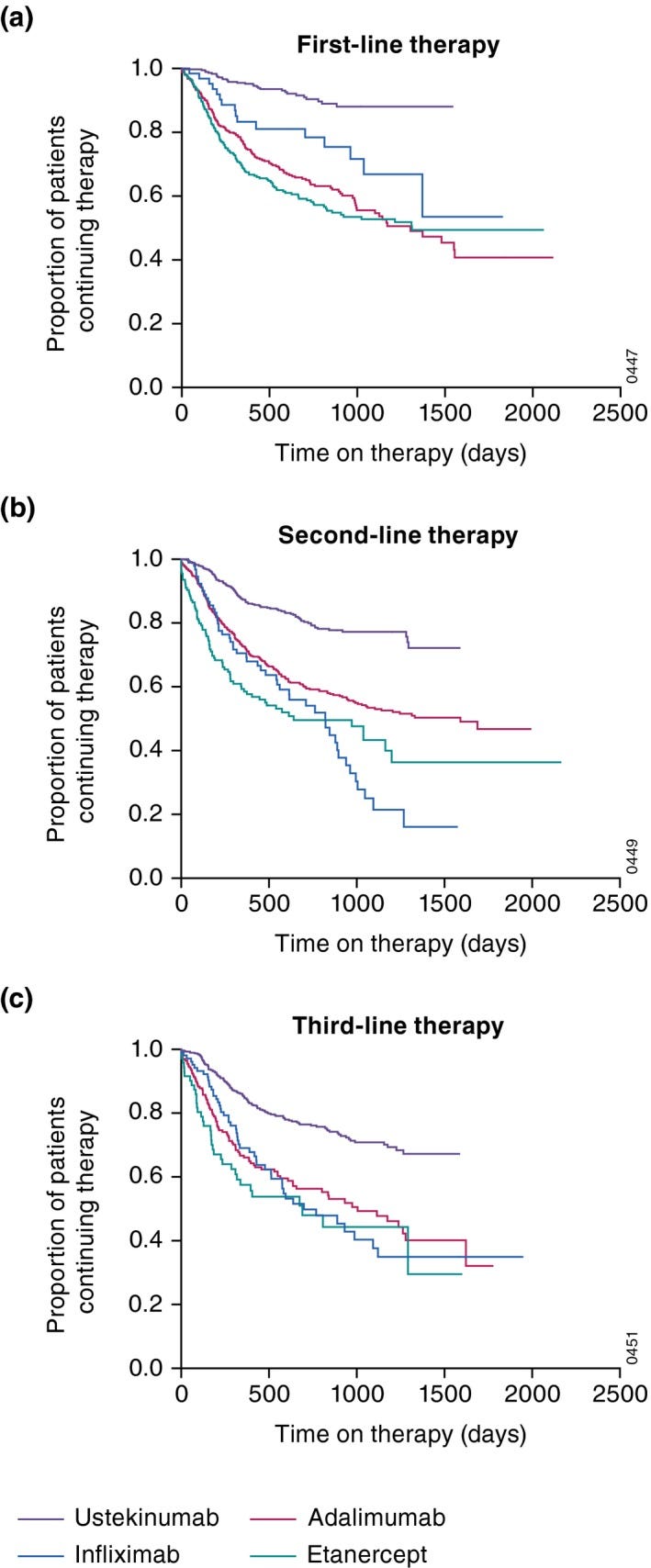
Drug Survival -
“Maintaining response to treatment is important in chronic conditions that require long-term treatment, such as psoriasis. It is known that drug survival decreases over time for all biologic therapies, mainly due to lack/loss of efficacy, eventual remission and side effects, depending on the study. Drug survival of biologics, defined as the time from initiation to discontinuation of a particular treatment, is well documented in several registries and cohort studies [2–4].”
Drug survival was an issue is 22% of respondents who’d discontinued an oral treatment according to patient surveys. The following charts plot drug survival of biologic injections. Drug survival and the safety of piclidenoson were the two primary pitches made by Can-Fite’s management in this presentation (link is timestamped).
Convenience -
While oral medications can obviously be taken at home, the impact on lifestyle is not zero. The text of image doesn’t cleanly fit inside this post, but simply read the “ongoing monitoring” section of this table and you’ll understand. It may be convenient to physically take an oral pill, but the baggage that comes along with them certainly isn’t. The only one which doesn’t require any sort of ongoing lab monitoring is apremilast, but patients are warned they should monitor themselves for depression & suicidal ideations.
Injectables can be administered by a patient at their home.
Now this isn’t an overly burdensome inconvenience to a patient, as these drugs are administered at frequencies spanning weeks to months. Infusions are moderately worse: infliximab is infused over a course of a two-hour duration + short term monitoring, and ustekinumab for an hour. Obviously, these will usually require a person going into a clinic versus self-infusion at home.
NB-UBV phototherapy also has its challenges as it relates to administration.
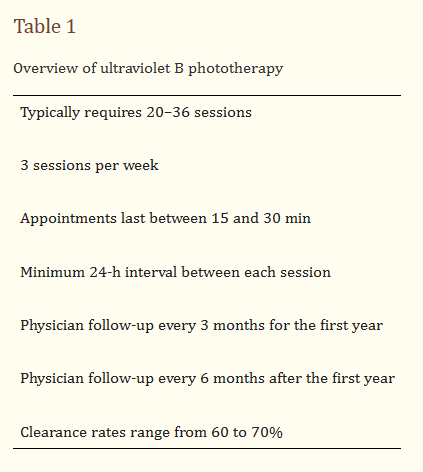
“Typically, patients with moderate-to-severe psoriasis require 20–36 sessions of NB-UVB phototherapy at a frequency of three sessions per week to see a significant improvement in their skin [9]. Each phototherapy appointment usually lasts about 15 min, although some patients may require up to 30 min, if more time preparing for phototherapy is needed. A minimum of 24 h is required between each session.”
In addition, “If a patient does not receive UVB phototherapy for a period of 12–20 days, the dose is decreased by at least 25% to prevent skin from burning. A 21- to 27-day break requires a 50% reduction in dose. 28 or more days off requires the patient to start the regimen again.”
Largely phototherapy is administered in a clinic, though it’s possible to purchase machines to perform the procedure at home. Insurance coverage of the devices varies.
Patient testimonial related to phototherapy who reached out curious about my discussions on piclidenoson.
Notes on cost of treatment -
Generally speaking, the pricing of these treatments is fairly intuitive.
Oral medications (with some exceptions detailed below) are less expensive than phototherapy, which is less expensive than biologics.
Combination of treatments
Psoriasis Therapy Prevalence for moderate to severe plaque psoriasis (published 2015)
EMERGENT COMPETITORS
SOTYKTU® (deucravacitinib) - this product is owned by Bristol-Myers Squibb Co. It was approved last year in September and is the latest drug to be approved for moderate to severe plaque psoriasis. The clinical efficacy of the product appears quite good - slightly less than 60% of patients reach PASI-75 and 10% to 14% achieved a PASI score reduced by 100% (PASI 100) response at week 16.
On this same note, CADTH (Canada's drug and health technology agency) recommended that sotyktu not be reimbursed by public drug plans for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. They describe their rationale for the decision in the link above. Their reasoning is fairly concerning because it’s largely attributed to the drugs inferiority against biologics, which is a hurdle rate that piclidenoson simply isn’t going to cross. The primary clinical edge piclidenoson has over sotyktu relates to its side effects, so should similar guidelines be followed by CADTH for piclidenoson once approved, they wouldn’t recommend the product for reimbursement.
Things to monitor
One of more my recent posts involved a recent discovery surrounding piclidenoson by the Telethon foundation.
There’s not much to update in terms of what happened here. They presented at Bio Japan 2023, but unfortunately meetings weren’t recorded.
However, we’re not totally in the dark…
Can-Fite estimated the value of this to be worth approximately 100M USD on the US market should the product were to make it through human testing. This estimate indirectly answers one of my prior questions: That is - can a drug which was developed for a different, far more common disease, be reimbursed at rates typical for rare disease should a discovery such as this be made and then make it to market?
Can-Fite’s market estimate seems to suggest they believe you can, as fewer than 750 people in the US have Lowe syndrome and 750 prescriptions of oral medication for plaque psoriasis certainly wouldn’t cross $100M USD in annual revenue.
In addition, as I noted in the prior post, all of the pre-requisite human safety testing has been done and this is for a rare condition which has no existing treatment options in terms of pharmaceuticals. Thus, the pipeline for testing of the product will be highly abbreviated. In terms of a timetable, it’s hard to pin down an exact date. But I would guess the outcome of this testing would be relevant to cipher prior to the end 2028.
PRODUCT RISKS
In terms risk for piclidenoson, there are three major risks as I see it at this time.
Regulatory risk - This is straightforward. The drug has not been approved and it may never be approved. However, I think the risk that it’s rejected for safety issues is basically nonexistent at this point. Should the drug initially fail to meet regulatory standards, it will likely have something to do with either efficacy or manufacturing of the product.
Market risk - There are dozens of treatment options available for plaque psoriasis. It’s a highly competitive market with dozens of entrants who’re well-funded and flush with cash to advertise their product to a greater capacity than Cipher Pharmaceuticals can afford. Further, insurance reimbursement of drugs is of critical importance for market adoption. Given CADTH recommended against the reimbursement of sotyktu (which is an oral medication) because of its inferiority against biologics (which are injections), this bodes poorly.
Development risk - This one is also straightforward: Can-Fite is not a well-capitalized company. They are burning cash, and their balance sheet is in rough shape (they very recently issued warrants to raise capital). Until recently, they only had a few quarters of runway left - which isn’t long enough to go to market for CF-101 in Canada given recruitment is ongoing. I suspect Can-Fite will simply continue to rely on capital markets through dilutive raises in the meantime.
I do not hold a position with the issuer such as employment, directorship, or consultancy.
I hold a material investment in the issuer's securities.
Note for readers: Thank you again for your support. I love doing this. If I was able, I would make this more than simply a hobby. I look forward to continuing to share additional research in the future.
Until next time,
-Left























Would love to see a summary at the end for people like me with adhd.