Statistical insignificance to the primary endpoint on a dosing response
This one's going to hurt
The FDA almost always requires replication of statistical significance in two P3 trials for registration of a product to be sold in America.
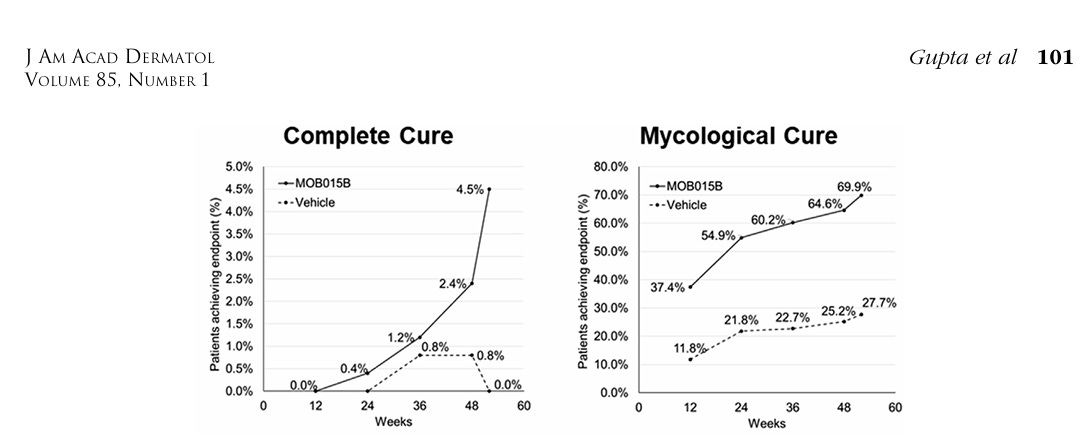
That being said, it appears MOB-015 did not meet its primary endpoint for statistical significance to placebo with a complete cure value of 1.5% vs 0% versus vehicle. To make matters worse, Bayer’s seemingly totally uninterested in their product now.
Anyways…
We’ll only see what’s to truly come from Moberg Pharma only in the future. I look forward to seeing the full set of data released in the future to better understand what has happened in this trial. A blanket mycological cure value of 25% and a complete cure value of 1.5% doesn’t really tell you the full story until you see how the non-control group has behaved across the set of who was recruited into it. The behavior and what happened across the set of patients with varying degree of nail involved recruited into the trial matters quite a lot in my opinion.
Moberg stepping away from America to focus on the EU is only rational in this circumstance. It’s hard to say what Bayer’s motivations really are because they do not speak of a complete cure value or mycological cure value for what they sell with their OTC brand.
Amir Tavakkol isn’t someone that’s dumb. He’s actually the exact opposite of someone that’s dumb. He’s highly intelligent and meticulous with everything. Those who had the chance to speak with him know this is true, and he’s their CSO. Anna didn’t make that trial design, Amir and Anders would have.
The label in Sweden tells you to avoid getting your feet wet for a number of hours after application because terbinafine will precipitate when exposed to too much water. It says the getting your feet wet part right on Allderma’s website. This is because the invention of concern for Moberg is how much terbinafine they can deliver and how long it takes the terbinafine in MOB-015 to precipitate out of the nail.
That trial design never said to not shower and the behavior of the nail is different with different frequency of application. Now I threw people into a company that has an extremely long history of very odd behavior that extends well beyond just the last 5 years, and for that I’m sorry if you got hurt because of it.
I took the full bore of the drawdown that’s going to come from this one and I earned this drawdown.
Moberg Pharma’s first and second phase 3’s demonstrates efficacy of a product that’s ideal for the treatment of nail fungus. In spite of the low complete cure value that began to skyrocket during the 4wk washout, there’s no other product that currently exists which can even fit these claims onto a 48wk once daily label.
The hurdle for statistical significance to the primary endpoint of antifungal nail topical is a relatively low bar to step over (it’s basically non-existent), but it seems that the dream of finally getting MOB-015 out to the world is still falling by the wayside.
In spite of the massive dosing reduction at play here, I thought they would handily step over this bar because again statistical significance with these products almost doesn’t exist. Hopefully Cipher Pharmaceuticals makes a good call here and tries to get the entirety of North America out of them to fit into their new dermatology business.
I’ll leave comments open on this one. I’m a little frustrated (probably not as frustrated as many of you), but it’s because what I see and what the majority of other people see are two entirely different things.





Great summary! Regardless of the outcome, the process here was near flawless - thank you for that.
So cipher could still get Us licensing rights and go rx instead of otc correct?