For some reason a few years ago, this guy was in a 4-person group chat with me talking about ClearPoint all day every day. You can still see the remnants of the brief past on his public feed. He’s an immunologist at a research university and he became very interested in doing intratumoral convection enhanced delivery of monoclonal antibodies into glioblastomas. Unfortunately, he’s no longer active on that site and I haven’t talked to him in years. But some of his story remains with mine.
So, what’s a monoclonal antibody?
Beyond already leaning into his specialty, I believe he was inspired by prior issues that are cut from the same cloth. Now I’m finding that I can’t at all be creative with language and have anyone understand me, so here’s the problem of old:
Without use of gadoteridol and or feedback of some kind as to the progression of the infusion to the surgeon, you basically just have a guy with a garden hose pumping in a therapeutic to some anatomical region of interest... Sure, you have a stereotactic headframe and guidance so a surgeon could conceive of a trajectory path to advance the catheter in with pre-operative imaging. But without seeing that infusion take place in real time you’re not actually seeing it happen. The snapshot of a post operative MRI contrast enhanced image is only the photo finish to the race. You don’t have the opportunity to actually see or correct for any mistakes during the race, they just happen as you’re driving down the road blindly… There are so many papers out there from this little gang of people and a central theme of theirs involves target coverage maximization.
This is really where it started for ClearPoint as it stands today. The historical listing sits inside a company that has changed quite dramatically after Joe Burnett came into the picture.
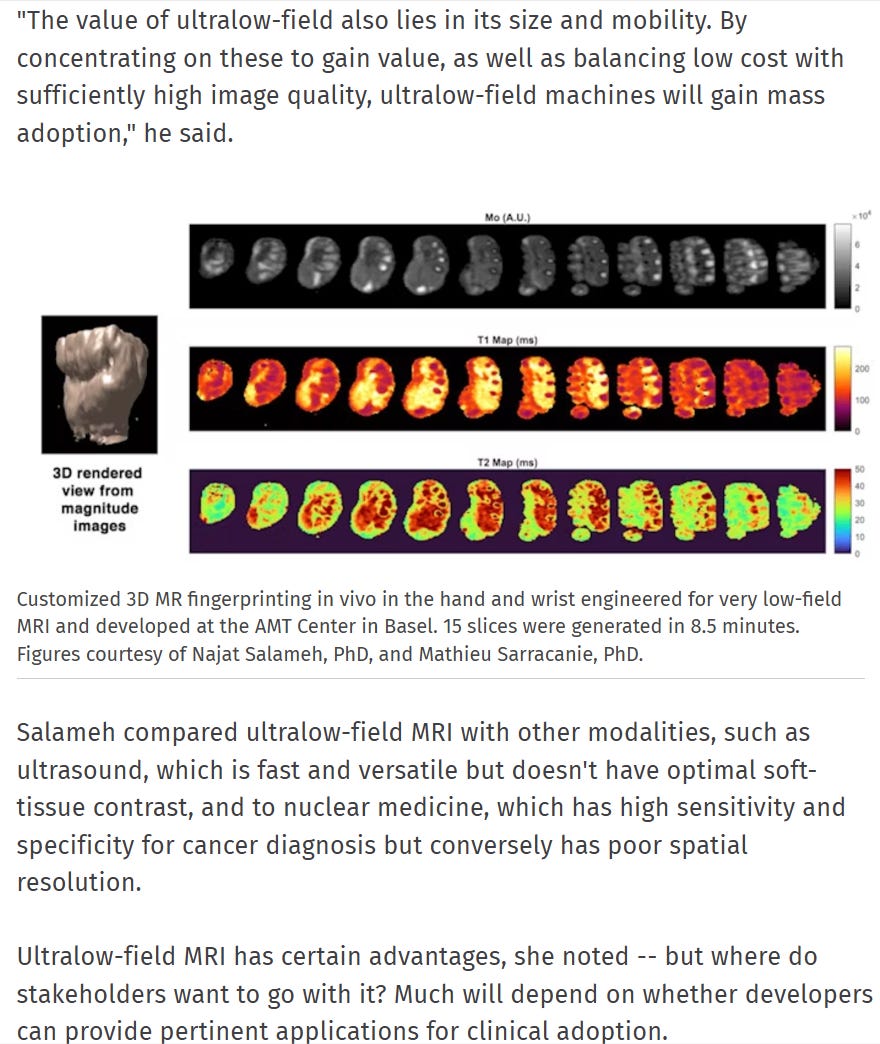
The set of tools required to harness the full capacity of this new wave of therapeutics into neuro was only completed by ClearPoint within the last year of time. I’ve long worried about issues of scaling these procedures up, but they’re doing everything that they can. I encourage anyone that’s reading this to look into some of the low-fi MRI work (that’s less than 1 Tesla coil) ongoing at ClearPoint. I won’t talk about it here but below is a brief summary of the give and take.
I’m going to keep this short but you’re probably going to need to look some things up.
While it sounds haughty and arrogant and ridiculous because it’s never been done (especially coming out of the mouth of someone with zero medical training), ClearPoint Neuro’s first real big break is going to be within Huntington’s disease.
Within just a few more years, we’re going to see some data back that changes the outlook of Huntington’s disease forever. It may come from more than just UniQure biopharma but AMT-130’s product works. The reason it works involves not just how that therapy is designed but how it is physically delivered. The microvasculature of the brains endothelial cells are very, very tightly conjoined. Their hydraulic conductivity to water flux is similar to that of slate and clay. One of the things that helps overcome this and get AMT-130 into the nucleus of as many target cells as they can involves a positive pressure infusion. What you are doing by connecting the therapy to a pump is turning bulk flow of a fluid driven by very small differential pressures into a guided convective flow. The result is a much, much better distribution profile of the drug that extends radially from the tip of the cannula.
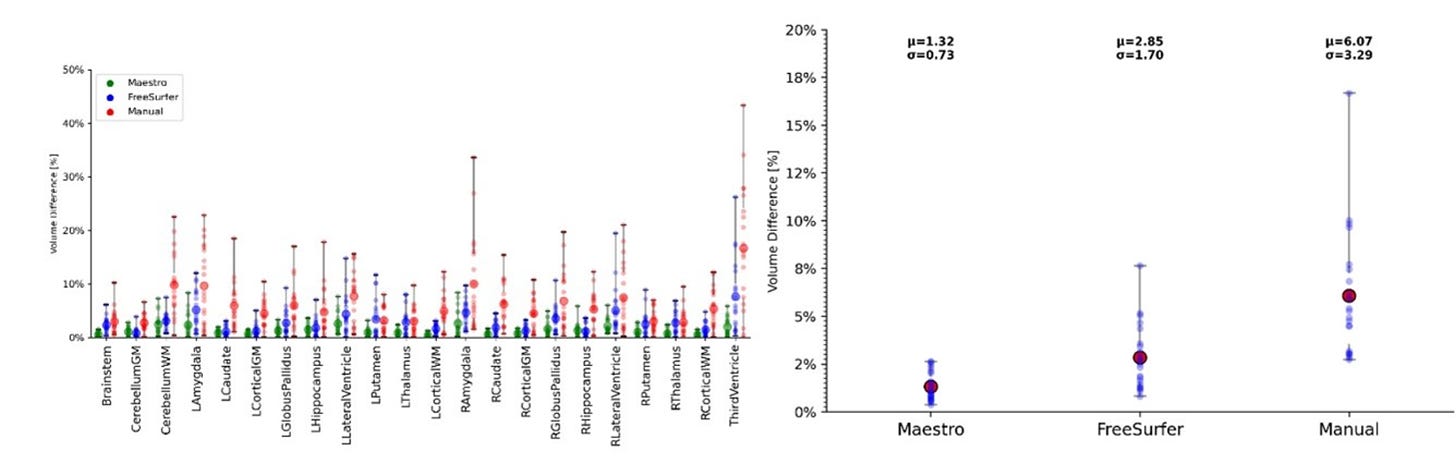
ClearPoint’s Maestro was the final piece of software that they really needed. More than anything, its development concerns placating the interests of insurance companies due to an extremely tight band of control on how much of a therapeutic you need and what the infusion looked like over the course of a surgery.
Now with the ingredients of their set of tooling, time and the very hard work of well-intentioned researchers, things are going to boil over into making an extremely high hit rate for translating experimental clinical products into commercial ones. It’s much easier seeing this today versus a few years ago because there’s a barrage of early-stage clinical news coming out of these biopharma companies that ClearPoint’s connected to.
I’m writing this because I need to. Your support is appreciated.
But I do want you to know that the anger I feel from the response of people due to a mere lowering of expectations from Moberg’s label isn’t something I’m ever going to easily let go. That’s the ideal antifungal nail topical medication. You can’t even make a better one without discovering an antifungal that’s better than terbinafine. The reasons for why aren’t easily understood without a little leg work though.